How To Machine Wash Dry Clean Only Clothes
Dry out cleaning is any cleaning process for wearable and textiles using a solvent other than h2o.
Dry cleaning even so involves liquid, but wearing apparel are instead soaked in a water-free liquid solvent, tetrachloroethylene (perchloroethylene), known in the industry as "perc", which is the most widely used solvent. Culling solvents are 1-bromopropane and petroleum spirits.[i]
Most natural fibers tin be washed in water only some synthetics (e.g., viscose, lyocell, modal, and cupro) react poorly with water and must exist dry-cleaned.[2]
History [edit]
Dry out cleaning originated[3] with American entrepreneur Thomas 50. Jennings. Jennings referred to his method as "dry scouring".
French dye-works operator Jean Baptiste Jolly[4] [a] developed his ain method using kerosene and gasoline to make clean fabrics.[4] He opened the first dry-cleaners in Paris in 1845.[6]
Flammability concerns led William Joseph Stoddard, a dry out cleaner from Atlanta, to develop Stoddard solvent (white spirit) as a slightly less combustible culling to gasoline-based solvents. The use of highly combustible petroleum solvents caused many fires and explosions, resulting in government regulation of dry out cleaners. After World War I, dry cleaners began using chlorinated solvents. These solvents were much less flammable than petroleum solvents and had improved cleaning ability.[ commendation needed ]
Shift to tetrachloroethylene [edit]
By the mid-1930s, the dry cleaning industry had adopted tetrachloroethylene (perchloroethylene), or PCE for short, equally the solvent. It has splendid cleaning power and is nonflammable and compatible with almost garments. Considering it is stable, tetrachloroethylene is readily recycled.[ane]
Infrastructure [edit]
Dry cleaning businesses, from the perspective of the customer, are either plants or drib shops. A plant does on-site cleaning. A drop store receives garments from customers, sends them to a large found, and then has the cleaned garment returned to the store for drove by the client. The turnaround time is longer for a drib shop than for a local plant. All the same, running a constitute requires more than piece of work for the business organisation owner. Since 2022, in some markets, spider web apps have been used to schedule depression-toll home delivery for dry cleaning.[7]
This cycle minimized the risk of fire or dangerous fumes created by the cleaning procedure. At this time, dry cleaning was carried out in two unlike machines—one for the cleaning process, and the second to remove the solvent from the garments.
Machines of this era were described as vented; their drying exhausts were expelled to the atmosphere, the same as many mod tumble-dryer exhausts. This non only contributed to environmental contagion but also much potentially reusable PCE was lost to the temper. Much stricter controls on solvent emissions have ensured that all dry cleaning machines in the Western world are now fully enclosed, and no solvent fumes are vented to the atmosphere.[ citation needed ] In enclosed machines, solvent recovered during the drying process is returned condensed and distilled, so information technology can be reused to clean farther loads or safely disposed of. The majority of modernistic enclosed machines as well comprise a computer-controlled drying sensor, which automatically senses when all detectable traces of PCE have been removed. This system ensures that merely small amounts of PCE fumes are released at the cease of the cycle.
Mechanism [edit]
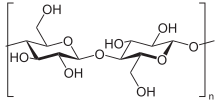
Structure of cellulose, the master constituent of cotton wool. The many OH groups demark water, leading to swelling of the fabric and leading to wrinkling, which is minimized when these materials are treated with tetrachloroethylene and other dry out cleaning solvents.
In terms of mechanism, dry cleaning selectively solubilizes stains on the article. The solvents are non-polar and tend to selectively extract compounds that cause stains. These stains would otherwise only dissolve in aqueous detergents mixtures at high temperatures, potentially damaging fragile fabrics.
Non-polar solvents are also good for some fabrics, especially natural fabrics, as the solvent does not interact with any polar groups inside the fabric. Water binds to these polar groups which results in the swelling and stretching of proteins within fibers during laundering. Also, the bounden of water molecules interferes with weak attractions within the fiber, resulting in the loss of the fiber's original shape. Afterwards the laundry cycle, water molecules volition dry off. Nevertheless, the original shape of the fibers has already been distorted and this commonly results in shrinkage. Not-polar solvents prevent this interaction, protecting more delicate fabrics.
The usage of an effective solvent coupled with mechanical friction from tumbling finer removes stains.
Process [edit]

A mod dry cleaning machine with touchscreen and SPS control, manufacturer EazyClean, blazon EC124, photo taken prior to installation

Serial 3 Dry cleaning machine with PLC control, manufacturer, BÖWE Material cleaning Germany
A dry-cleaning car is similar to a combination of a domestic washing car and clothes dryer. Garments are placed in the washing or extraction chamber (referred to as the 'basket' or 'drum'), which constitutes the core of the motorcar. The washing chamber contains a horizontal, perforated drum that rotates within an outer shell. The beat out holds the solvent while the rotating drum holds the garment load. The handbasket capacity is between about 10 and 40 kg (22 to 88 lb).[ citation needed ]
During the wash cycle, the sleeping room is filled approximately i-third full of solvent and begins to rotate, agitating the clothing. The solvent temperature is maintained at thirty degrees Celsius (86 degrees Fahrenheit), as a higher temperature may damage it. During the launder cycle, the solvent in the sleeping room (commonly known as the 'cage' or 'tackle box') is passed through a filtration chamber and and then fed back into the 'cage'. This is known as the cycle and is continued for the wash duration. The solvent is then removed and sent to a distillation unit consisting of a boiler and condenser. The condensed solvent is fed into a separator unit of measurement where any remaining h2o is separated from the solvent and then fed into the 'clean solvent' tank. The ideal catamenia rate is roughly 8 liters of solvent per kilogram of garments per minute, depending on the size of the machine.
Garments are also checked for strange objects. Items such as plastic pens may dissolve in the solvent bath, damaging the textiles. Some textile dyes are "loose" and will shed dye during solvent immersion. Fragile items, such equally feather bedspreads or tasseled rugs or hangings, may be enclosed in a loose mesh purse. The density of perchloroethylene is around 1.7 g/cm3 at room temperature (seventy% heavier than water), and the sheer weight of absorbed solvent may cause the material to fail nether normal force during the extraction cycle unless the mesh purse provides mechanical support.
Not all stains can be removed by dry out cleaning. Some need to be treated with spotting solvents — sometimes by steam jet or by soaking in special stain-remover liquids — before garments are washed or dry cleaned. Also, garments stored in soiled condition for a long time are difficult to bring back to their original colour and texture.
A typical wash bike lasts for viii–fifteen minutes depending on the type of garments and degree of soiling. During the first three minutes, solvent-soluble soils dissolve into the perchloroethylene and loose, insoluble soil comes off. It takes 10–12 minutes after the loose soil has come off to remove the ground-in insoluble soil from garments. Machines using hydrocarbon solvents crave a wash bike of at to the lowest degree 25 minutes because of the much slower charge per unit of solvation of solvent-soluble soils. A dry cleaning surfactant "soap" may likewise exist added.
At the cease of the wash cycle, the automobile starts a rinse bicycle where the garment load is rinsed with freshly distilled solvent dispensed from the solvent tank. This pure solvent rinse prevents discoloration acquired by soil particles being captivated back onto the garment surface from the 'dirty' working solvent.
Afterwards the rinse bike, the machine begins the extraction process, which recovers the solvent for reuse. Modern machines recover approximately 99.99% of the solvent employed. The extraction cycle begins by draining the solvent from the washing chamber and accelerating the basket to 350–450 rpm, causing much of the solvent to spin free of the fabric. Until this fourth dimension, the cleaning is done in normal temperature, as the solvent is never heated in dry cleaning process. When no more solvent can be spun out, the car starts the drying cycle.
During the drying wheel, the garments are tumbled in a stream of warm air (60–63 °C/140–145 °F) that circulates through the basket, evaporating traces of solvent left later the spin cycle. The air temperature is controlled to foreclose heat impairment to the garments. The exhausted warm air from the machine then passes through a chiller unit of measurement where solvent vapors are condensed and returned to the distilled solvent tank. Mod dry out cleaning machines utilise a closed-loop organization in which the chilled air is reheated and recirculated. This results in high solvent recovery rates and reduced air pollution. In the early on days of dry out cleaning, large amounts of perchloroethylene were vented to the atmosphere because it was regarded as inexpensive and believed to be harmless.

Many dry cleaners place cleaned wearing apparel inside thin clear plastic garment bags
After the drying cycle is consummate, a deodorizing (aeration) cycle cools the garments and removes further traces of solvent, by circulating cool outside air over the garments and then through a vapor recovery filter made from activated carbon and polymer resins. After the aeration cycle, the garments are clean and ready for pressing and finishing.
Solvent processing [edit]

A Firbimatic Saver Series. This automobile uses activated clay filtration instead of distillation. Information technology uses much less energy than conventional methods.
Working solvent from the washing chamber passes through several filtration steps before it is returned to the washing chamber. The get-go footstep is a button trap, which prevents small objects such as lint, fasteners, buttons, and coins from inbound the solvent pump.
Over fourth dimension, a thin layer of filter cake (called "muck") accumulates on the lint filter. The muck is removed regularly (unremarkably once per day) and then candy to recover solvent trapped in the muck. Many machines use "spin disk filters", which remove the muck from the filter past centrifugal force while information technology is dorsum washed with solvent.
After the lint filter, the solvent passes through an absorptive cartridge filter. This filter, which contains activated clays and charcoal, removes fine insoluble soil and not-volatile residues, along with dyes from the solvent. Finally, the solvent passes through a polishing filter, which removes any soil not previously removed. The clean solvent is then returned to the working solvent tank. Cooked pulverisation residue is the name for the waste product material generated past cooking down or distilling muck. It will comprise solvent, powdered filter material (diatomite), carbon, not-volatile residues, lint, dyes, grease, soils, and water. The waste sludge or solid residue from the still contains solvent, h2o, soils, carbon, and other not-volatile residues. Used filters are some other form of waste matter every bit is waste product water.
To enhance cleaning power, pocket-sized amounts of detergent (0.5–1.five%) are added to the working solvent and are essential to its functionality. These detergents emulsify hydrophobic soils and keep soil from redepositing on garments. Depending on the machine's design, either an anionic or a cationic detergent is used.
Symbols [edit]
The international GINETEX laundry symbol for dry cleaning is a circle. Information technology may have the letter P within it to indicate perchloroethylene solvent, or the letter F to indicate a flammable solvent (Feuergefährliches Schwerbenzin). A bar underneath the circle indicates that only mild cleaning processes is recommended. A crossed-out empty circle indicates that an item should not exist dry cleaned.[viii]
-

Professional cleaning symbol
-

Dry make clean, hydrocarbon solvent only (HCS)
-

Gentle cleaning with hydrocarbon solvents
-

Very gentle cleaning with hydrocarbon solvents
-

Gentle cleaning with PCE
-

Very gentle cleaning with PCE
-
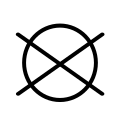
Do not dry clean
Solvents used [edit]
Perchloroethylene [edit]
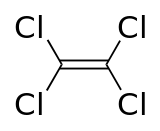
Perchloroethylene (PCE, or tetrachloroethylene) has been in use since the 1930s. PCE is the most common solvent, the "standard" for cleaning performance. It is a highly constructive cleaning solvent. It is thermally stable, recyclable, and has low toxicity. It can, notwithstanding, cause colour bleeding/loss, peculiarly at higher temperatures. In some cases it may damage special trims, buttons, and chaplet on some garments. It is better for oil-based stains (which account for about 10% of stains) than more common water-soluble stains (coffee, vino, blood, etc.). The toxicity of tetrachloroethylene "is moderate to low" and "Reports of human injury are uncommon despite its wide usage in dry out cleaning and degreasing".[9]
Hydrocarbons [edit]
Hydrocarbons are represented by products such as Exxon-Mobil's DF-2000 or Chevron Phillips' EcoSolv, and Pure Dry out. These petroleum-based solvents are less aggressive but also less constructive than PCE. Although combustible, chance of fire or explosion can be minimized when used properly. Hydrocarbons are however pollutants. Hydrocarbons retain about 10-12% of the market place.

A modern dry cleaning machine for use with diverse solvents
Trichloroethylene [edit]
Trichloroethylene is more ambitious than PCE only is very rarely used. With superior degreasing properties, it was oftentimes used for industrial workwear/overalls cleaning in the past. TCE is classified every bit carcinogenic to humans past the United States Environmental Protection Agency.[10]
Supercritical CO2 [edit]
Supercritical CO2 is an alternative to PCE; however, it is inferior in removing some forms of grime.[11] Additive surfactants amend the efficacy of COii. [12] Carbon dioxide is virtually entirely nontoxic. The greenhouse gas potential is besides lower than that of many organic solvents.
The dry cleaning process involves charging a sealed chamber which is loaded with clothes using gaseous carbon dioxide from a storage vessel to approximately 200 to 300 psi. This step in the process is initiated as a precaution to avoid thermal daze to the cleaning chamber. Liquid carbon dioxide is then pumped into the cleaning chamber from a split storage vessel by a hydraulic, or electrically driven pump (which preferably has dual pistons). The pump increases the pressure of the liquid carbon dioxide to approximately 900 to 1500 psi. A separate sub-cooler reduces the temperature of the carbon dioxide by 2 to 3 degrees Celsius beneath the boiling signal in an effort to foreclose cavitation which could lead to premature deposition of the pump. [13]
Consumer Reports rated supercritical COii superior to conventional methods, just the Drycleaning and Laundry Institute commented on its "fairly low cleaning ability" in a 2007 report.[fourteen] Supercritical CO2 is, overall, a balmy solvent which lowers its ability to aggressively assail stains.
One deficiency with supercritical CO2 is that its electric conductivity is depression. As mentioned in the Mechanisms department, dry cleaning utilizes both chemical and mechanical properties to remove stains. When solvent interacts with the fabric'due south surface, the friction dislocates dirt. At the aforementioned time, the friction also builds up an electric charge. Fabrics are very poor conductors and and then usually, this build-up is discharged through the solvent. This discharge does not occur in liquid carbon dioxide and the build-upwards of an electrical charge on the surface of the fabric attracts the dirt dorsum on to the surface, which diminishes its cleaning efficiency. To compensate for the poor solubility and electrical conductivity of supercritical carbon dioxide, research has focused on additives. For increased solubility, 2-propanol has shown increased cleaning furnishings for liquid carbon dioxide as information technology increases the ability of the solvent to dissolve polar compounds.[15]
Machinery for apply of supercritical CO2 is expensive—up to $90,000 more a PCE car, making affordability difficult for small-scale businesses. Some cleaners with these machines keep traditional machines on-site for more heavily soiled textiles, but others find establish enzymes to be equally effective and more environmentally sustainable.
Other solvents: niche, emerging, etc. [edit]
For decades, efforts have been made to replace PCE. These alternatives have non proven economical thus far:
- Stoddard solvent – flammable and explosive, 100 °F/38 °C wink signal
- Chlorofluorocarbon-113 (Freon-113), a CFC. Now banned as ozone-unfriendly.
- Decamethylcyclopentasiloxane ("liquid silicone"), chosen D5 for brusk. It was popularized by GreenEarth Cleaning.[16] It is more expensive than PCE. It degrades within days in the environs.
- Dibutoxymethane (SolvonK4) is a bipolar solvent that removes water-based stains and oil-based stains.[17]
- Brominated solvents (due north-propyl bromide, Fabrisolv, DrySolv) are solvents with higher KB-values than PCE. This allows faster cleaning, but tin damage some synthetic chaplet and sequins if not used correctly. Healthwise, there are reported risks associated with nPB such as numbness of fretfulness.[xviii] The exposure to the solvents in a typical dry cleaner is considered far below the levels required to cause any take a chance.[19] Environmentally, it is approved by the U.S. EPA. Information technology is among the more than expensive solvents, but information technology is faster cleaning, lower temperatures, and quick dry times.
See also [edit]
- Cloth restoration
- List of laundry topics
- Moisture cleaning
Notes [edit]
- ^ In some sources incorrectly[v] referred to every bit "Jolly-Belin"
References [edit]
- ^ a b David C. Tirsell "Dry out Cleaning" in Ullmann'due south Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. doi:10.1002/14356007.a09_049
- ^ Hunter, Jennifer (22 May 2022). "Dry Cleaning Your Wool Sweaters? Don't Bother". The New York Times . Retrieved 30 May 2022.
- ^ Johnson, Shontavia. "America's e'er had black inventors – even when the patent system explicitly excluded them". The Conversation . Retrieved 2021-06-19 .
- ^ a b Oladele Ogunseitan (3 May 2022). Green Health: An A-to-Z Guide. SAGE Publications. pp. 135–. ISBN978-i-4522-6621-three.
- ^ Ancliffe Prince (1965). Laundering and Cleaning: Yesterday, To-day, and To-morrow. Iliffe Technical Publications.
In Britain America the discovery was for long attributed to a supposed Paris tailor by proper name of Jolly-Belin [...] Actually the discoverer of drycleaning was not named Jolly-Belin but Jean-Baptiste Jell
- ^ Reed Business Information (13 February 1986). New Scientist. Reed Business Data. pp. 33–. ISSN 0262-4079.
- ^ Lee, Sunny (ane October 2022). "The uncertain future of your neighborhood dry out cleaner". The Outline . Retrieved 2019-10-11 .
- ^ "Professional textile care symbols". GINETEX - Swiss Association for Fabric Labelling. Archived from the original on 2022-05-28. Retrieved 2013-07-18 .
- ^ Due east.-L. Dreher; T. R. Torkelson; Thousand. K. Beutel (2011). "Chlorethanes and Chloroethylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01. ISBN978-3527306732.
- ^ EPA Releases Final Health Cess for TCE [1] September 2022. Accessed 2022-09-28.
- ^ "Dry-cleaning with CO2 wins award [Science] Resources". Resource.wur.nl. 2022-10-12. Archived from the original on 2022-03-12. Retrieved 2013-03-14 .
- ^ "How tin can we use carbon dioxide every bit a solvent?". Contemporary topics in school science. Retrieved 2016-08-29 .
- ^ "Liquid/supercritical carbon dioxide/dry cleaning organisation". 1993-12-06. Retrieved 2021-01-02 .
- ^ Drycleaning and Laundry Institute. "The DLI White Newspaper: Key Information on Industry Solvents." The Western Cleaner & Launderer, August 2007.
- ^ Usa 5784905, Townsend, Carl W.; Chao, Sidney C. & Purer, Edna M., "Liquid carbon dioxide cleaning system employing a static dissipating fluid", published 1998-07-28
- ^ Tarantola, Andrew. "At that place'due south a Better Way to Dry out Make clean Your Dress". Gizmodo . Retrieved 2016-08-29 .
- ^ Ceballos, Diana Chiliad.; Whittaker, Stephen G.; Lee, Eun Gyung; Roberts, Jennifer; Streicher, Robert; Nourian, Fariba; Gong, Wei; Broadwater, Kendra (2016). "Occupational exposures to new dry cleaning solvents: High-flashpoint hydrocarbons and butylal". Periodical of Occupational and Environmental Hygiene. 13 (ten): 759–769. doi:ten.1080/15459624.2016.1177648. PMC5511734. PMID 27105306.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ "Take chances EVALUATION 1-Bromopropane" Archived 2022-11-06 at the Wayback Automobile July 2003. Accessed 2022-Jan-22
- ^ Azimi Pirsaraei, Due south. R.; Khavanin, A; Asilian, H; Soleimanian, A (2009). "Occupational exposure to perchloroethylene in dry out-cleaning shops in Tehran, Iran". Industrial Health. 47 (2): 155–nine. doi:10.2486/indhealth.47.155. PMID 19367044.
External links [edit]
- Gamble Summary provided by the United States Environmental Protection Bureau.
- NIOSH Safe and Health Topic: Drycleaning
Source: https://en.wikipedia.org/wiki/Dry_cleaning
Posted by: villegasunely1936.blogspot.com


0 Response to "How To Machine Wash Dry Clean Only Clothes"
Post a Comment